APPG Aesthetics Inquiry Recommendations
In 2019 an All-Party Parliamentary Group (APPG) was formed to represent the interests of the beauty, aesthetics and wellbeing sectors. One of its first tasks was to hold an inquiry into the current state of non-surgical aesthetics practices in the UK.
This began in Summer 2020 and the concluding report was published on 21 July 2021.
It took into account previous reports such as the Joint Council of Cosmetic Practitioners’ 10 Point Plan from 2020 and the 2013 Sir Bruce Keogh review.
Key findings from this APPG aesthetics inquiry revolve around the need for a minimum, defined standard of education, such as a Level 7 qualification, as well as a national register for all practitioners.
As the developers of and pioneers behind the Level 7 Diploma in Botox and Dermal Fillers, this is something Harley Academy has championed since its onset. We are, therefore, thrilled to have our educational standards validated in this way. However, there is still a lot of work to be done.
Whilst the findings have resulted in a number of recommendations being made, they stop short of requiring mandatory regulation or legislative change.
Here we take you through the crux of the report’s highlights so we can explore just how it can help shape the future of the UK’s aesthetics industry.
Key recommendations from the APPG aesthetics inquiry
• Framework
• Standards and qualifications (including introducing a minimum standard of training and qualification for all aesthetics practitioners)
• Regulation and enforcement (including making dermal filler a Prescription Only Medication)
• Ethics and mental health
• Insurance
• Social media and advertising
Framework
In order for the aesthetics industry to be able to become properly regulated, the APPG found that a framework needs to be put in place.
This should involve the Government legally defining what constitutes:
• “Medical-related” services
• “Elective aesthetic non-surgical cosmetic treatment”.
It advises these definitions should be based on how a patient accesses these services. For example, whether they are “self-elected, medically diagnosed or ancillary/referred”.
In order to make “informed policy decisions regarding the industry to support practitioners and protect consumers” it was noted that the Government should collect data on:
• Types of aesthetic treatments
• Numbers of practitioners
• Premises
• Training courses
• Complications.
Standards and qualifications
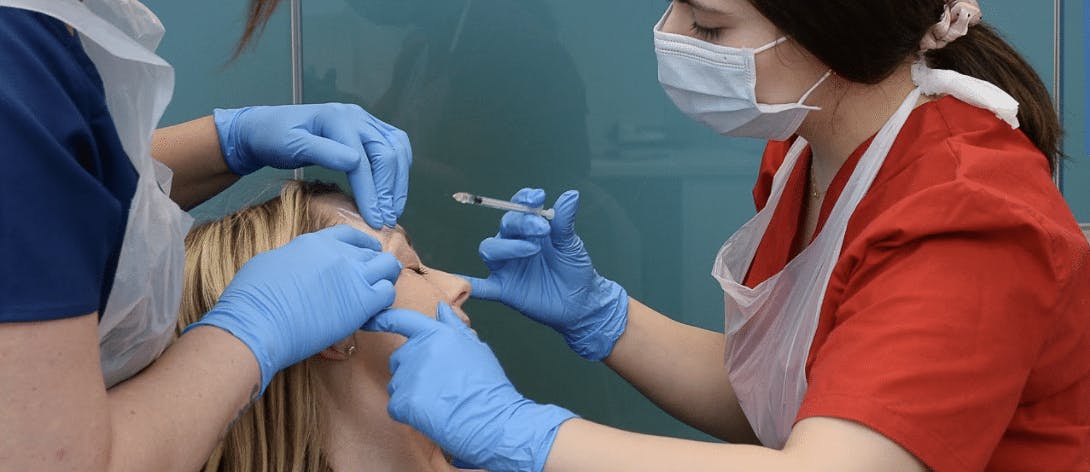
The lack of required or even consistent standards in both education and practice across the industry was acknowledged. Particular concerns about non-medical injectors were also noted.
Whilst the Inquiry did not go as far as to find that medical aesthetics should only be practised by medically-trained healthcare professionals – something many aesthetics practitioners believe strongly in – there was a “broad agreement that nationally regulated training and qualifications should be mandatory for new practitioners”.
The APPG revealed it had heard from a number of bodies and individuals concerned that non-medic beauty therapists should not be barred from entering aesthetics. However, a designated training and qualification framework was required to ensure these lay practitioners were properly equipped to practice some treatments alone, with others potentially being performed under the guidance of a medic.
Three recommendations were made by the APPG in respect of standards and qualifications.
“The Government must set national minimum standards for the training that all practitioners must be required to undertake to provide aesthetic non-surgical cosmetic treatments, based on the HEE and NOS standards. The aesthetics industry must work together to align and agree education and training frameworks. These frameworks must include annual CPD for all practitioners, medic and non-medic, to update their competencies and prove fitness to practice.”
“The Government must empower Ofqual to require regulated Awarding Organisations to evidence that their qualification curriculum is compliant with nationally set minimum standards, and all aesthetic practitioners should therefore be required to hold a regulated qualification in line with this. Ofqual must also ensure academic progression routes to regulated qualifications are available from a range of Awarding Organisations for all aesthetic practitioners.”
“On-site medical oversight must be mandatory for aesthetic non-surgical cosmetic treatments using Prescription-Only Medicines, where the treatments are performed under the oversight of the prescriber who has gained the accredited qualifications to prescribe, supervise and provide remedial medicines if necessary. An initial face-to-face consultation with the person providing the medical oversight (the prescriber) must also be mandatory prior to any treatment.”
The APPG acknowledging just how crucial it is for aesthetics practitioners to achieve a high standard of training and obtain a properly-regulated, accredited qualification is vindicating for many within the industry.
We know many Harley Academy trainees who are doing everything the right way – completing recognised Foundation Training, becoming licensed, insured practitioners and investing in their future via up-skilling, whether this is through undertaking the Level 7 Diploma or tailored one-to-one injectables mentoring sessions. They can feel deflated at the thought of having to compete against rogue practitioners whose expertise, products and safety cannot be assured but their prices are cheap and patients have not yet been sufficiently educated as to why this is.
It does feel as if the tide may be turning, especially as the UK media have been getting on board and now regularly run stories illustrating the desperate need for change across the aesthetics industry.
Regulation and enforcement
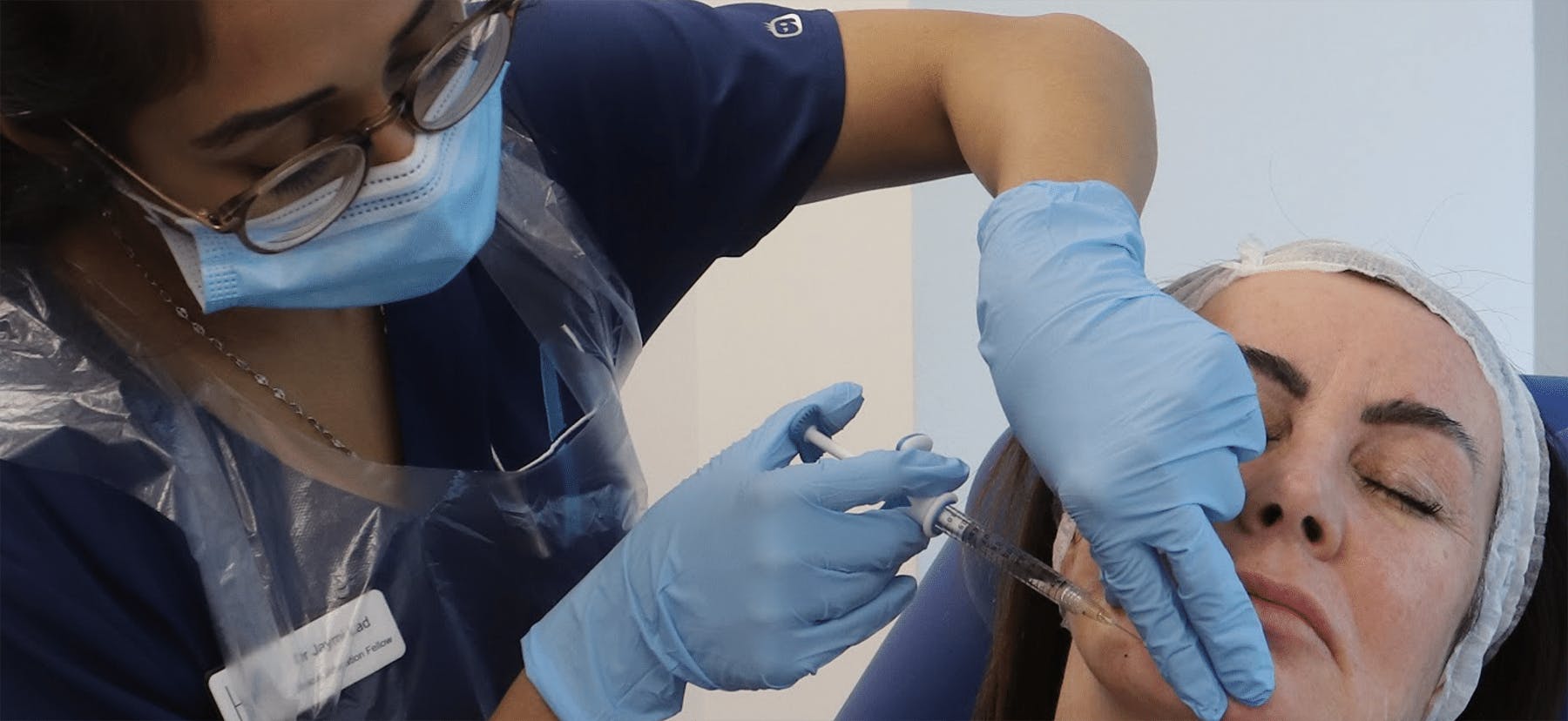
Cases were considered both for and against:
• Voluntary registration of aesthetics practitioners
• Mandatory registration of aesthetics practitioners
• Standards and requirements necessary for registration.
The APPG concluded that it is presently “not minded to recommend a mandatory register of practitioners and sees the priority for Government action to be putting in place nationally set standards and a framework for the required public safety, training and qualification of practitioners, which can be enforced via other avenues.”
It did have recommendations in other parts of this sphere though, especially with regard to:
• Developing a licensing framework for practitioners
• Ensuring licensing of aesthetics practitioners and procedures is future-proofed to avoid new services falling through the regulatory cracks
• Closing loopholes which make current systems unworkable, such as the inability to regulate home-based or mobile injectors
• Establishing a minimum standard of safety, training and qualification for all aesthetic practitioners.
Again, our core company values were echoed and upheld by the APPG as it underscored the need for industry-wide standards of education and qualifications, to be reinforced via a recommended licensing scheme.
The recommendations made in this section of the report were set out as follows….
“The APPG recommends that the Government introduces a national licensing scheme to govern the oversight of advanced aesthetic non-surgical cosmetic treatments such as botox, dermal fillers, PDO cogs and threads. It should consider amending the Local Government (Miscellaneous Provisions) Act 1982, or introducing such a scheme via new primary legislation as the most appropriate avenue to do so.”
“A national licensing scheme must be supported by a clear framework mandating the national minimum standard of public safety, training and qualifications for all practitioners. This should be developed with industry based on the HEE framework and NOS standards.”
“The Government must work with industry to develop guidance to underpin a national licensing scheme for advanced aesthetic non-surgical cosmetic treatments, as has been done with special procedures such as tattooing and piercing.”
Besides the recommendations for a national licensing scheme and minimum standards of training and qualifications, there was another headline recommendation in this section of the report.
The Inquiry found that dermal fillers should be reclassified from a medical device – available for purchase by non-medics – to a Prescription-Only Medication. This would mean both botox and fillers would only be available via a suitable prescriber following face-to-face consultation with the patient. This is intended to put a stop to virtual prescribing.
“The APPG recommends that dermal fillers be classified as a Prescription Only Medicine. In line with recommendation 5, on-site medical oversight must be mandatory for the administration of these products, and an initial face to face consultation with the person providing the medical oversight (the prescriber) must take place prior to any treatment. Dermal fillers must be performed under the oversight of a prescriber who has gained the accredited qualifications to prescribe, supervise and provide remedial medicines if necessary.”
Ethics and mental health
Professional ethics and the effects of advanced aesthetic non-surgical cosmetic treatments on patients’ mental health were considered.
The absence of mandatory mental health screenings for patients prior to undergoing aesthetics procedures was a major concern.
Age restrictions were part of this discussion, following the landmark ban on administering cosmetic injectables to under 18s which passed into law earlier this year.
These discussions resulted in a further three recommendations.
“The Government must work with the aesthetics industry on the development of psychological pre-screening tests to cover a range of broader psychological vulnerabilities, and make these mandatory prior to a consumer undergoing an aesthetic non-surgical cosmetic treatment.”
“Education on spotting at risk individuals, covering a broad range of psychological vulnerabilities, must be included in national minimum standards for the training that practitioners must be required to undertake to be qualified to deliver aesthetic non-surgical cosmetic treatments.”
“The Government must extend the legal ban on under 18s receiving botox or fillers to other invasive advanced aesthetic non-surgical cosmetic treatments including PDO cogs and threads.”
Insurance
The disconnect between the public’s assumption that individuals and businesses in the aesthetics industry are properly insured and the frequently uninsured reality was perturbing to the APPG.
Debates centred on the existing, woefully inadequate, system whereby there is no mandate that requires aesthetics practitioners to have any form of insurance. Insurance that is granted is not necessarily subject to any quality checks and a lack of requisite skill level, demonstrable by achieving a set qualification, makes this a minefield for anyone who does carry these out.
Even medically-trained healthcare professionals need to only attend a one-day course in order to obtain insurance and start practising immediately. It was recognised that this does not adequately prepare people to start injecting or performing other complex treatments such as PDO thread lifts.
The following recommendations were made to address these issues.
“The Government should require all practitioners to hold adequate and robust insurance cover and set an industry standard for the level of proven competence that is required to gain coverage. Any future national licensing scheme must also make this a requirement of holding a licence.”
“Practitioners must also be required to hold regulated qualifications for the aesthetic non-surgical cosmetic treatments they provide, alongside appropriate industry approved CPD training, to maintain and update their skills, knowledge and competence as part of annual insurance renewal, particularly as new treatments continue to emerge in the market.”
Social media and advertising

The APPG partnered with the Social Media APPG to address these widespread and rapidly growing problems. It also heard input from the Advertising Standards Authority (ASA).
Marked concerns that spurred the Inquiry into making recommendations included:
- False advertising of aesthetics training courses which mislead practitioners into thinking they are potentially more qualified than they are
- Misleading advertising of aesthetics treatments using undeclared influencer posts and/or filters which improve the appearance of results
- The effects of advertising cosmetic procedures on social media users’ body image and mental health
- Advertising of Prescription-Only Medications, such as botulinum toxin, on social media (which is illegal in the UK)
- Who is allowed to use certain terms to advertise their work online, including “qualified” and “skilled”
- Lack of recourse when rules are broken, resulting in toothless legislation and widely flouted advertising guidelines.
These led to the Inquiry’s last recommendations.
“Social media platforms must take more responsibility for curbing and censoring misleading advertisements and for the mental health impacts of promoting aesthetic non-surgical cosmetic treatments. As part of the Online Harms Bill, social media companies should be encouraged to publish specific policies on appropriate advertising of these treatments and act swiftly to take down any that breach those policies.”
“Advertising restrictions should be placed on dermal fillers and PDO cogs and threads in the same way they are imposed on botox as a Prescription Only Medicine.”
“We welcome the Government’s commitment to consult on the Online Advertising Programme later this year and urge them to specifically consider the promotion and sale of aesthetic treatments and training courses as part of this.”
Next steps…
Next steps for the APPG involve circulating their report across both government and the Opposition, gaining support from parliament to help implement their recommendations.
Whilst precise steps have not yet been announced, we believe there will be an announcement regarding the introduction of mandatory aesthetics training and qualification requirements before Spring 2022.
Harley Academy remains committed to upholding and promoting the highest standards in medical aesthetics training. We hope that positive change will be forthcoming on the back of this report and look forward to bringing you the latest developments on this, as they happen.
All information correct at the time of publication.












