MHRA Crackdown on Illegal Botox Suppliers: Up to 2-Years in Jail
The Medicine & Health Regulations Authority (MHRA) has announced new penalties for illegal botox suppliers.
New penalties of up to two years in prison have been announced as part of the MHRA’s bid to stop the use of illicit botulinum toxin products.
Here we outline the latest information and provide resources to help you ensure your neurotoxin products are genuine, appropriate and come from a reputable source.
We also explain how you can communicate the quality of the products you use to your patients, whilst keeping within the advertising regulations relating to botox.
Unlimited fines and prison sentences of up to two years
In a press release, dated 29 September 2025, the MHRA warned criminals ‘that they face prison as it cracks down on the illicit trade in unlicensed botulinum toxin products’.
Penalties of up to two years in prison and unlimited fines will be handed out to those caught ‘selling or supplying’ unlicensed botox. This is possible under the Human Medicines Regulations Act of 2012.
Criminal Enforcement Unit investigations
This action follows a wave of botulism outbreaks across England earlier in 2025. A total of 41 confirmed cases were reported between 4 June and 6 August 2025, across various regions in the Midlands, North-East and North of England.
Patients involved in outbreaks in Northumberland told the media they had been treated by a non-medic aesthetic practitioner, who they believed had used a counterfeit botox product. The injector later apologised.
The MHRA confirmed that its ‘Criminal Enforcement Unit has launched a number of criminal investigations following a spike in hospital admissions believed to be linked to the use of unlicensed botulinum toxin products’.
Does this MHRA crackdown apply to aesthetic practitioners or just product suppliers?
The MHRA press release is somewhat unclear on whether ‘supplier’ refers solely to those selling or otherwise providing the products.
However, we can confirm, after consultation with the JCCP - which references the two-year prison penalty in its prescribing guidance - that, in this context, ‘selling and supplying’ does refer to:
- Vendors or suppliers providing unlicensed product to practitioners; and
- Aesthetic practitioners ‘supplying’ the product to patients, by administering it during treatment.
The MHRA does note: ‘The Criminal Enforcement Unit has seen evidence that some sellers and practitioners – often untrained – are obtaining unlicensed botulinum toxin products illegally and offering injections in unsafe, unregulated settings’. This follows the June 2025 report from the Chartered Trading Standards Institute, which found aesthetic treatments being carried out in a range of unsuitable locations, including public toilets.
The MHRA press release also refers to ‘Anyone caught selling or supplying unlicensed botulinum toxin’ being eligible for penalisation.
For the avoidance of doubt, every clinician should be using genuine, licensed botulinum toxin products, purchased from authorised and properly licensed suppliers.
Fake botox versus unlicensed products
There are two key groups that problematic ‘toxin’ products can be grouped into:
- Unlicensed products
- Counterfeit products.
Often these are cheap, sold online or from unprofessional settings, and have poor quality packaging.
Supplying or using these products can result in fines, prosecution and/or professional sanctions. So, let’s explore the difference between fake and unlicensed toxins, and the MHRA’s approaches towards each.
What is meant by ‘unlicensed botulinum toxin products’?
As a prescription-only medication (POM), botulinum toxin is strictly regulated.
In the UK, a medicinal product is considered licensed once it’s received a marketing authorisation from the MHRA. This confirms it meets the requisite government standards for safety, quality and efficacy.
Essentially, if a neurotoxin does not have this MHRA approval, it’s considered unlicensed in the UK. This usually relates to toxin brands which may be approved in other countries.
Importing or using such products is considered illegal and unsafe, since they bypass the UK’s quality and safety checks. You can find a list detailing examples of licensed UK toxins below.
The MHRA treats unlicensed botox products as a regulatory issue and breach of the Medicines Act.
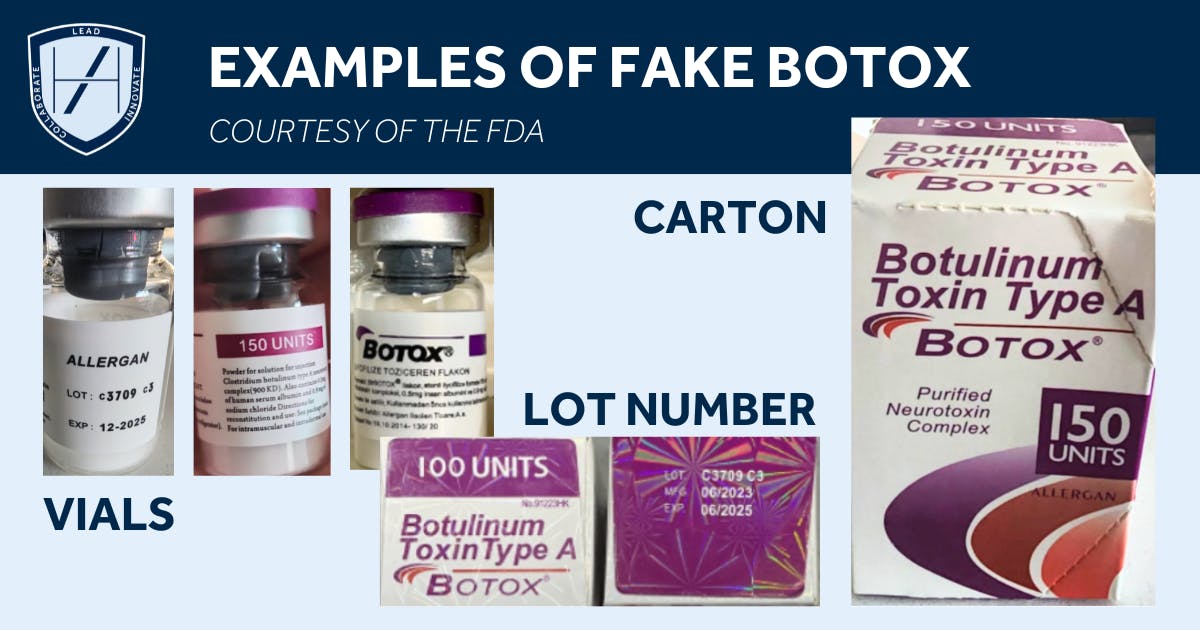
What constitutes a counterfeit botox product?
Counterfeit toxins are fake botox products packaged to look like genuine brands. The actual liquid, however, is not the genuine medication. Instead, it may contain undeclared, unsafe, or incorrect ingredients.
Fake medicines are incredibly dangerous and pose serious public health risks. As such, the MHRA treats their supply as a criminal offence under medicines legislation and trading standards law.
It can seize and destroy counterfeit stock, prosecute suppliers, and works with police and Border Force to stop imports.
Cases are often pursued as fraud, illegal supply of medicines, or public health endangerment.
For tips on how to ensure you’re purchasing authentic goods from a reputable supplier, read, How to Spot Fake Botox Products.
Licensed botulinum toxin products (UK)
In the UK, licensed botulinum toxin brands, which each have their own approved indications, include:
- Azzalure® (Galderma)
- Bocouture® (Merz)
- Botox® (Allergan)
- Dysport® (Ipsen x Galderma partnership)
- Nuceiva® (Evolus).
Once you’ve decided on the toxin you wish to purchase for use in your clinic, you must choose a reputable supplier to buy it from.
As you’re buying a POM, you should always purchase direct from the manufacturer or from an established pharmacy, with a verifiable Registered Pharmacy mark and number.
Our article detailing how to find and work with a prescriber also provides advice on how to check if a pharmacy is genuine.
How to communicate your botox product quality within the advertising rules
With the botulism outbreak news and word of these new penalties in the media highlighting this issue, it’s natural that patients will be concerned. As an ethical and responsible aesthetic practitioner, we’re sure you’ll want to reassure your patients, and not just in person.
However, as botulinum toxin is a prescription only medication, marketing of this product is highly restricted. You cannot mention ‘botulinum toxin’, ‘botox’ or any synonym, such as ‘anti-wrinkle treatment’ in any marketing under the advertising guidance. This includes:
- Social media posts of any form, including Instagram Stories or live broadcasts
- Your website, except for on the pricelist
- Emails, Newsletters, WhatsApp or other formats for promotional messages
- Physical marketing materials, such as brochures, flyers or posters.
The Committee of Advertising Practice (CAP), has clear information on what is and what is not permissible in relation to the promotion of botox, on a dedicated section of its website. We highly encourage you to familiarise yourself with these rules.
How to address patients' botox product concerns in your marketing
So, how can you address patient concerns, if you cannot mention ‘botox’ or in any way allude to ‘wrinkle reduction’ treatments? Sarah says, there is one golden rule of thumb: focus on promoting your expertise, your standards and your consultation - not the individual treatment.
“You can absolutely talk about, for example, ‘the risks of counterfeit or illegal treatments’, and the effects these can have. Just be careful to ensure the tone is educational and that you do not mention the medication or brand name.
“The inclination is often to publish a post that says, for example ‘The MHRA is cracking down on illegal botox. Rest assured, at [Clinic Name] we only use premium, licensed Botox for your injections’. This will also often use a link in bio to a Botox webpage. But this wording and linking is not permissable.
“A better way to do this is to say instead, ‘The MHRA is cracking down on illegal cosmetic injectable products. Rest assured, at [Clinic Name] we take your safety seriously and only use premium products from reputable suppliers.’ You can then promote your professional background, qualifications, reputation and invite people to book a consultation if they want to find out more.”
She continues, “You can talk generally about ‘products’ and ‘injectables’ as long as you’re not clearly referring to toxin only. Outside of that, the focus should be on you as a practitioner and what you bring to the table, not the product itself or its actions.
“The more transparent your content can be about your clinical setting, your qualifications, and your patients’ experiences - including reviews, not just testimonial videos or ‘before and after’ photos - the better. This is where you can truly put your professional background as a healthcare professional to work, alongside your aesthetics training qualifications.
“Social media posts demonstrating these aspects help your audience to form a picture of you, get to know you and build trust at a distance.”
You can find out more about this in our article, How Can You Advertise Toxin Treatments Legally in the UK?.
A reminder on UK aesthetics regulation
2025 has certainly seen unprecedented movement when it comes to tackling the ‘Wild West’ of aesthetic practice in the UK.
In June 2025, Scotland published its proposals for aesthetics regulation, including the introduction of ‘supervision’ for non-medic injectors. The Scottish government is aiming to have draft legislation ready for review in May 2026.
England lagged behind, finally publishing the responses to the 2023 public consultation relating to its initial regulatory proposals, in August 2025. These confirm plans to introduce a licensing scheme for aesthetic practitioners and their premises.
Work on the definitions and requirements - including educational standards - needed to establish this type of legislative framework is said to be underway. The next public consultation is said to be taking place in ‘early 2026’ but no date has yet been set.
As we wait, this work being carried out by the MHRA does at least give hope that some of the industry’s public safety issues are being addressed.
All information correct at time of publication
Download our full prospectus
Browse all our injectables, dermal fillers and cosmetic dermatology courses in one document
By submitting this form, you agree to receive marketing about our products, events, promotions and exclusive content. Consent is not a condition of purchase, and no purchase is necessary. Message frequency varies. View our Privacy Policy and Terms & Conditions
Attend our FREE open evening
If you're not sure which course is right for you, let us help
Join us online or in-person at our free open evening to learn more
Our Partners













STAY INFORMED
Sign up to receive industry news, careers advice, special offers and information on Harley Academy courses and services


