How Can You Advertise Toxin Treatments Legally in the UK?

Find out how to promote botox treatments to your audience within the UK guidelines. Plus we outline what the penalties are for non-compliance - including fines, prison sentences and being struck off.
We’re frequently asked how UK aesthetics practitioners can legally advertise toxin treatments. It’s understandable that new injectors would be confused as many injectors use different methods – whether legal or not. Even the most cursory glance at Instagram or TikTok provides a wealth of misinformation.
Here we list some of the most frequently asked questions regarding advertising botox.
We’ve provided answers which outline the basic regulations for how to promote your aesthetics treatments within the guidelines. At the end of this article, we’ve also included some useful resources to help you understand what the rules are. This includes a video regarding advertising botulinum toxin injections, in particular.
HOW CAN I ADVERTISE BOTOX TREATMENTS?
The answer to this is crystal clear for UK aesthetic practitioners: you can’t.
Botulinum toxin is a prescription-only medication (POM) and is, therefore, banned from being advertised in any medium. You cannot refer to the medication directly or indirectly, nor to the use of toxin for treatment
This means you cannot mention that you offer botox in any form of marketing, whether it’s paid advertising or not.
The only exception to this is that you may list the name of your treatment, for example Botox (3 areas) alongside the cost, on your price list. This does come with additional stipulations, such as the fact that you may not add any further information to this, such as detailing what the treatment does. Nor can you link any information about relevant concerns to your price list, nor share your price list on social media. You should always direct your audience to book a consultation before they are shown any price list.
With the exception of your price list, you cannot mention that you offer toxin, nor the effects treatments can offer in any patient-facing material.
This includes (but is not limited to):
- Social media, including hashtags
- Websites
- Online directory listings
- Posters
- Flyers
- Mailshots or newsletters
- Digital communications eg. WhatsApp or text messages
- Logos
- Business cards
- Email signatures
- Reviews and/or testimonials shared or published by you or by a third party on your behalf.
It also includes the context of celebrity endorsements and competitions when marketing botox. These are considered poor practice in the context of medical treatments.
Be aware that you also cannot publish ‘Before & After’ botox treatment images or photographs of toxin products.

But if I call it 'anti-wrinkle treatment', that's OK, right?
Wrong. You cannot legally refer to botulinum toxin injections under any name or synonym.
We know this is widely done, however the Advertising Standards Authority (ASA) is currently cracking down on this. The ASA has recently been upping its actions in banning social media accounts that promote botox treatments.
Also, remember that when you’re seeing adverts or posts on social media, they may be from other countries which have different rules and regulations. For example, in the USA toxin brands advertise on TV!
Here in the UK, none of the below commonly used words and phrases that refer to botulinum toxin treatments are legally permitted.
- Anti-wrinkle treatment / injections
- Brand names of toxin, eg. Bocouture, Daxxify, etc
- Medical names of toxin, eg. onabotulinumtoxina
- Botox
- Botulinum toxin
- BTX
- B*tox
- Tox
- Toxin
- Wrinkle reduction / relaxing / removal.
How can I let people know that I offer toxin treatments?
You can promote a consultation in order to manage a concern. Ideally this should be done in the context of various treatment options that may be suitable or unsuitable for an individual.
Secondly, you are permitted to talk about concerns you may be able to help your patients address in your marketing and communications. What you must not do, however, is state that you would use botox to treat these, or give them any expectations as to the results they may achieve. As such, the best way to ‘advertise’ botox treatments is to focus on the consultation.
Example of an appropriate approach

If you wanted to advertise upper face toxin treatments, this example shows a good approach. It keeps to the patient’s concerns and the fact that you can help. This also directs them to get more information from a qualified professional. It doesn’t try to sell them a prescription-only medication, nor does it promise or allude to any potential benefits or results.
Example of an inappropriate approach
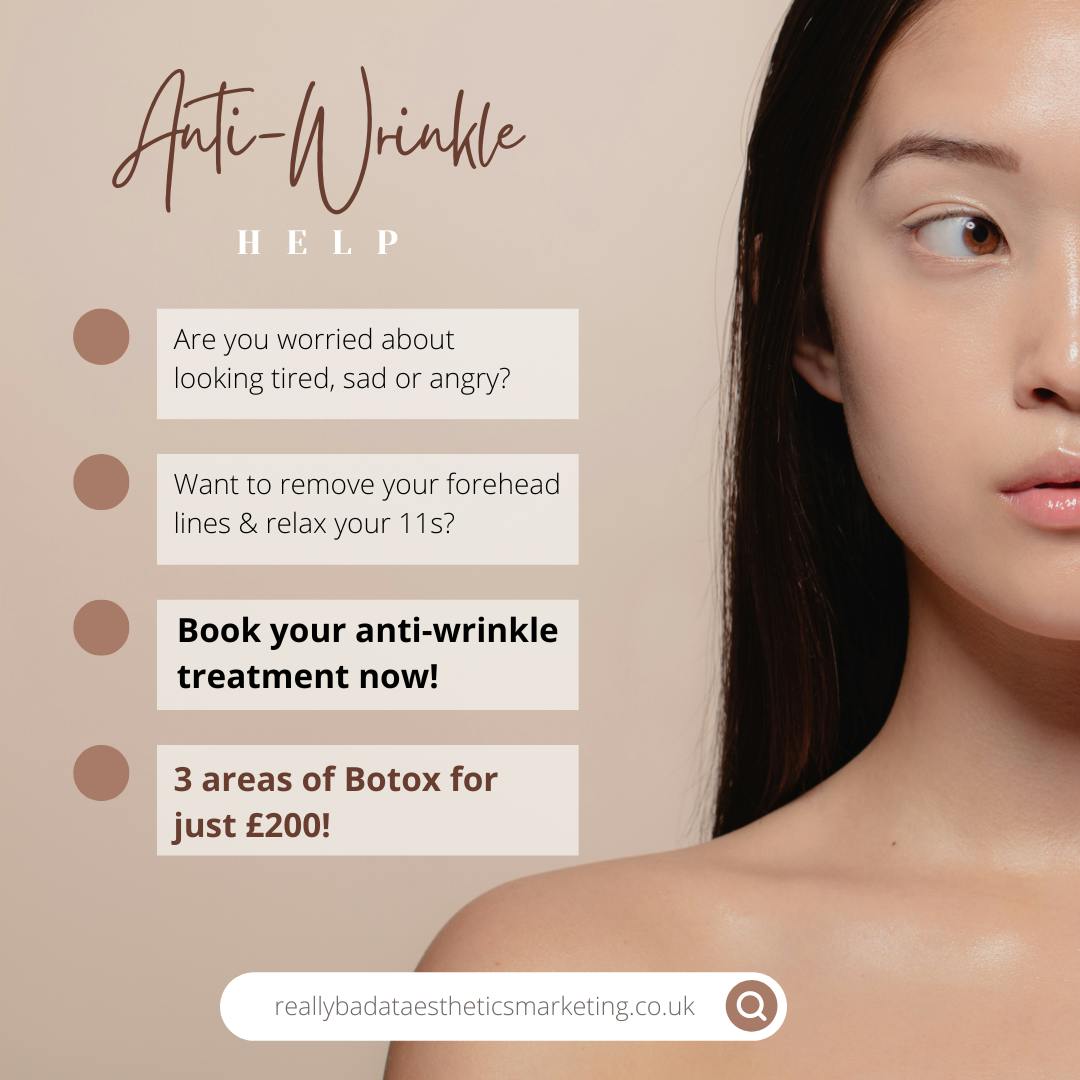
In contrast, whilst the second line is permissible, this type of approach should be avoided. Not only does it mention prescription medication both directly (Botox) and indirectly (anti-wrinkle treatment), but it also mentions potential effects of this treatment. Furthermore, it also gives the impression that the treatment is a bargain, leading patients to potentially make a decision based on incentive pricing, which is also forbidden.
Understanding the UK rules for advertising aesthetics treatments
To help you fully understand the rules for advertising aesthetics treatments, including botox, the ASA and the Committee of Advertising Practice Ltd. (CAP) have published a series of videos.
Below you can watch the video specific to the ‘advertising’ of botox by aesthetics practitioners. Again, remember that when they say “advertising”, this does not mean just paid advertising – it means any form of promotion, as explained above.
You can also watch the full range of CAP “Bitesize” videos on the ASA website, which we highly recommend you do. This will help you to fully understand what is and is not permitted when promoting your practice. It features a number of topics, including some specifically relating to your use of images and photo production, social responsibility, misleading claims, and price promotions.
Penalties for non-compliance - from fines to jail time
The Human Medicines Regulations 2012 prohibit any advertisement “likely to lead to the use of a prescription-only medicine” being directed at the public. This covers all POMS, so includes botulinum toxin, lidocaine and hyaluronidase, for both 'direct and indirect' promotions.
In relation to social media, it should be noted the rules apply to accounts belonging to the aesthetic practitioner, not just specific clinic accounts.
The CAP regulations outlined above are additional to this and are specific to advertising. This means that, when advertising treatments such as botox, you are both breaching the CAP Code and breaking the law.
As of 2025, the ASA, which enforces these CAP rules, is using custom AI technology to identify instances of non-compliance. If you're found to be in breach following an investigation, a formal ruling against you will be made. This information will be publicly available and be available for search engines and AI tools such as ChatGPT to use in query responses.
Usually, for single breaches, you'll be asked to amend or remove any offending adverts, social media posts, website pages or other content. For on-going or more widespread rule-breaking, harsher penalties come into play.
Whilst the ASA is limited in which sanctions it can impose, it can refer you to the MHRA and your professional body. The MHRA may pursue statutory action, including enforcement notices, prosecutions, and criminal sanctions. These can go as far as a sentence of up to two years in prison.
CAP advises, "You could be listed on our non-compliant online advertiser page. It is likely we will refer you to representative or professional association of which you may be a member, to be assessed under their own rules or code of conduct. This could result in your membership being suspended or your licence to practice being revoked."
Nurses may be sanctioned or struck off if they do not stick to the rules, according to a January 2026 update from the Nursing & Midwifery Council (NMC).
All information correct at the time of publication.
Download our full prospectus
Browse all our injectables, dermal fillers and cosmetic dermatology courses in one document
By submitting this form, you agree to receive marketing about our products, events, promotions and exclusive content. Consent is not a condition of purchase, and no purchase is necessary. Message frequency varies. View our Privacy Policy and Terms & Conditions
Attend our FREE open evening
If you're not sure which course is right for you, let us help
Join us online or in-person at our free open evening to learn more
Our Partners













STAY INFORMED
Sign up to receive industry news, careers advice, special offers and information on Harley Academy courses and services

