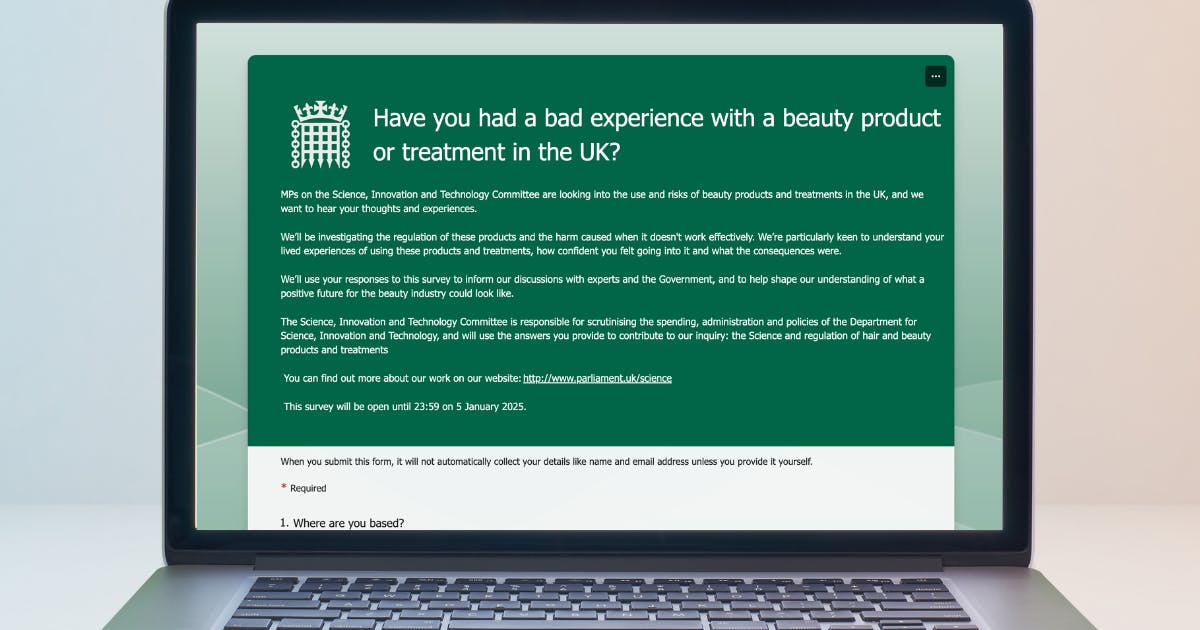
Government Inquiry Into Aesthetic Treatments: Patient Survey Live
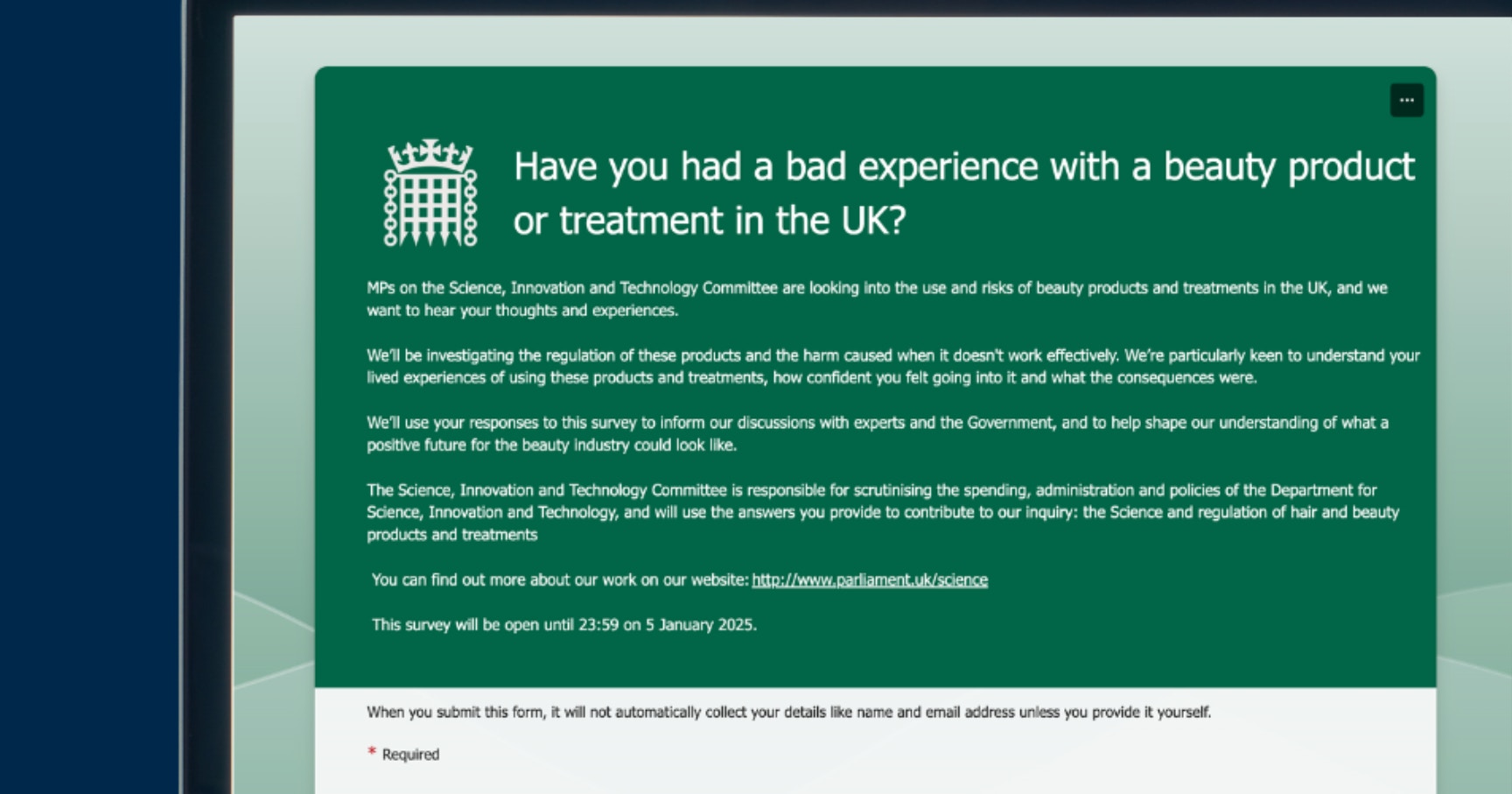
The research needed to ensure proper aesthetics regulation continues with a government inquiry into aesthetic procedures and beauty products. A new patient survey is now live, requesting feedback by 5th January 2026.
On 2nd December 2025, a UK inquiry into the ‘science and regulation of hair and beauty products’ was launched. This is headed up by the government’s Science, Innovation and Technology committee.
Patient input needed to inform aesthetics regulation
The next public consultation on regulating non surgical cosmetic procedures is expected in early 2026. However, in the meantime, the government is seeking specific input from members of the public who use hair and beauty products and/or have aesthetic treatments.
The team advises that these can ‘can cause harm to consumers if they contain unregulated ingredients or if they are improperly administered’.
It further notes that this inquiry ‘will respond to recent research which has led to concerns about the current scientific evidence base and regulation for these products, as well as the required training and qualifications of specialists performing treatments.’
One of its specific aims outside of the non surgical cosmetic procedures and skincare realm is to explore the potential toxicity of certain afro-textured hair products.
Insights into beauty products and aesthetic treatments
Some of the key insights the inquiry hopes to gain from this patient survey, are:
- ‘How do UK regulators assess scientific evidence to ensure the safety of beauty products and treatments? Are existing processes effective, including for individuals with diverse hair and skin types?
- Is there sufficient transparency in how scientific evidence is used in regulatory decisions for hair and beauty products to ensure public trust?
- How well are consumers informed about the potential harms of beauty products and treatments? How can this be improved?
- For beauty products and treatments where the evidence suggests there may be risks, what studies need to take place to better understand potential harms for users, including in the longer term?
- How effective are existing training and qualifications requirements for individuals who administer beauty treatments in minimising harm to their recipients? How could this be improved?
- What changes (regulatory, legal or otherwise) could ensure that consumers are better protected from and aware of the risks of potentially harmful beauty products and treatments?’
The goal is to ensure all treatments are fit for purpose, and that treatments, products and practitioners meet public standards. This includes understanding the level of beauty therapy or aesthetics training and qualifications your practitioner has achieved, and what the public wants to see in this regard.
Inquiry follows BABTAC evidence to Scottish Parliament
This UK information-gathering exercise follows evidence provided to the Scottish Parliament, also on 2nd December 2025, by BABTAC.
BABTAC is the British Association of Beauty Therapists and Cosmetologists. This organisation is similar to the Joint Council for Cosmetic Practitioners (JCCP), but for non-medics. Just like the JCCP, it also works hard to ensure proper regulation of the non surgical cosmetic sector.
Lesley Blair MBE, CEO and Chair of BABTAC and Victoria Brownlie MBE, Chief Policy and Sustainability Officer, British Beauty Council, both attended this parliamentary meeting. They highlighted ‘concern that the proposed Scottish Non-surgical Procedures and Functions of Medical Reviewers (Scotland) Bill varies substantially to the Scottish Government’s Consultation Response, as laid out in June 2025.’
You can refresh your memory on the information available on aesthetics regulation for Scotland and England in our articles on the subject. These can be found in the Regulation section of our Aesthetic Medicine Articles homepage.
Key concerns raised on behalf of non-medics
BABTAC’s main issues, in representing their membership of beauty professionals, were summarised by them, as follows:
- ‘The bill is a useful starting point but needs much more detail and structure to keep pace with an evolving industry and restore proper oversight
- A clear regulatory framework is essential for both healthcare and non-healthcare practitioners, providing consumer education, practitioner accountability and parity across the sector
- Non-healthcare practitioners currently have no formal recourse or accountability if something goes wrong, and introducing this would raise standards and protect the public
- Regulated, standardised qualifications and a risk-based hierarchy of procedures are vital to create national benchmarks, raise industry standards and eliminate unsafe practices in what has become an unregulated Wild West while still protecting professional fit for purpose beauty businesses (as determined by the groupings originally agreed upon in the Bill)
- Scotland’s current Bill is too broad and lacks detail, particularly around training, education, oversight, and clarity on which healthcare professionals are qualified to supervise treatments
- While many practitioners are highly competent, non-healthcare individuals are not legally able to access and administer the prescription medication needed to safely manage complications from injectables.’
‘Injectables should only be carried out by medical practitioners’‘
Sharing news of this meeting, BABTAC confirmed in a social media post that its ‘stance remains clear’ in that:
- ‘Injectables should only be carried out by medical practitioners’‘
- ‘Qualified, insured beauty professionals should be permitted to deliver lower-risk advanced aesthetics in a regulated, safe environment.’
To this end, the BABTAC team were concerned about variations between the proposed regulatory classifications for cosmetic injectables.
Notable variances between Scotland and England for injectables
Something we have previously expressed concern about was also brought to the Scottish Parliament’s attention by the BABTAC representatives. This relates to the different categorisations of treatments between UK countries.
As Lesley Blair, MBE, explains, “As drafted, the [proposed Scottish Non-surgical Procedures bill places all aesthetic treatments, from superficial peels to injectables, into a single category. This contrasts with the risk-based system used in England, where treatments are categorised using a red, amber and green model.
“We firmly believe that injectables should only be administered by suitably qualified medical practitioners. At the same time, we support a framework that enables beauty therapists to perform advanced aesthetic treatments such as microneedling and chemical peels, provided they hold fit-for-purpose qualifications and adequate insurance.”
She also noted, “We need a framework that applies to both healthcare and non-healthcare practitioners and is based on regulation that is proportionate and appropriate to the level of risk of each treatment. Clear licensing, consumer education and defined boundaries would help ensure that practitioners work within their qualifications and capabilities, and that the public understands where to go and what standards to expect.”
It’s important for the UK to have a joined-up approach when it comes to regulation, to prevent further ‘border-hopping’ issues. These are currently happening where young teenagers wanting injectable treatments but are underage in England, are ‘hopping’ over to Wales, for example. Whilst England has a ban on consultations and cosmetic procedures for under 18s, this is not true of the rest of the UK.
UPDATE: On 13th December a survey of 2,000 UK adults was released by Cosmetic Surgery Solicitors. It polled respondents on matters relating to aesthetics regulation and safety. The survey found that 82% were in favour of UK-wide legislation to ban under 18s from accessing cosmetic injectables.
How you can get involved in shaping aesthetics regulation now
Want to help determine the future of aesthetics regulation in the UK? The best steps you can take, as a medical professional and aesthetic practitioner, now, are:
- Look out for opportunities to feed back to dedicated government inquiries and consultations. We share these here on the Harley Academy website as soon as information becomes available. You can also keep an eye on websites for the Department of Health & Social Care (DHSC), the JCCP, BABTAC and the Science, Innovation & Technology Committee
- Write to your MP with any concerns you’d like them to address locally, or raise in parliament
- Educate your peers and patients on how they can get involved, too - the more voices, the merrier!
Complete the patient survey by 5th January 2026
One thing you can do right now is to complete this latest government survey. If you’re a UK resident and use haircare or skincare products, or have any beauty or aesthetic treatments, your feedback is requested.
Fill out the simple form by 23:59 on 5th January 2026 in order for your responses to be counted.
Please encourage your patients to participate, too! The more feedback the government receives, the better their aesthetics regulation and licensing scheme proposals should be.
Whilst progress remains slow, we’re hopeful for more meaningful regulatory outcomes in 2026. As always, we’ll keep you informed and up-to-date on the latest developments.
In the meantime, if you’re looking to formalise your aesthetic medicine credentials, or are a healthcare professional wanting to get started in aesthetics, we can help. From botox and filler courses for beginners, to our Ofqual-regulated qualification, the Level 7 Diploma in Cosmetic Injectables, plus various Masterclasses, we have something for everyone at our JCCP-approved academies in London and Manchester.
Our Courses team is happy to help you find the best aesthetics training solution for your needs. Simply book a call back for personalised advice.
All information correct at time of publication
Download our full prospectus
Browse all our injectables, dermal fillers and cosmetic dermatology courses in one document
By submitting this form, you agree to receive marketing about our products, events, promotions and exclusive content. Consent is not a condition of purchase, and no purchase is necessary. Message frequency varies. View our Privacy Policy and Terms & Conditions
Attend our FREE open evening
If you're not sure which course is right for you, let us help
Join us online or in-person at our free open evening to learn more
Our Partners













STAY INFORMED
Sign up to receive industry news, careers advice, special offers and information on Harley Academy courses and services