Does Botox Wear Off Quicker for Ozempic Patients?
A new study explores how quickly the effects of botox wear off in patients taking GLP-1 inhibitors, such as Ozempic.
The rise of weight loss drugs - GLP-1 receptor agonists such as Ozempic (semaglutide) and Mounjaro (tirzepatide) - has been stratospheric. As an aesthetic practitioner, you’re increasingly likely to encounter patients using these medications.
We’ve already written about the side effect known as ‘Ozempic face’ and what injectors need to be aware of in this respect.
Now there’s a further update for this cohort, as a recent study explored whether botox wears off quicker for patients using weight loss drugs.
Here we review that research, its findings and the practical implications for injectors. Read to the end for a clinical case scenario to see how you’d react!

Botulinum toxin mechanism and timeline of effect
Before addressing this study and any interactions, let’s revisit the fundamentals of botulinum toxin type A (BoNT-A) for cosmetic use.
It’s produced by Clostridium botulinum and functions by blocking acetylcholine release at the neuromuscular junction, causing muscle relaxation.
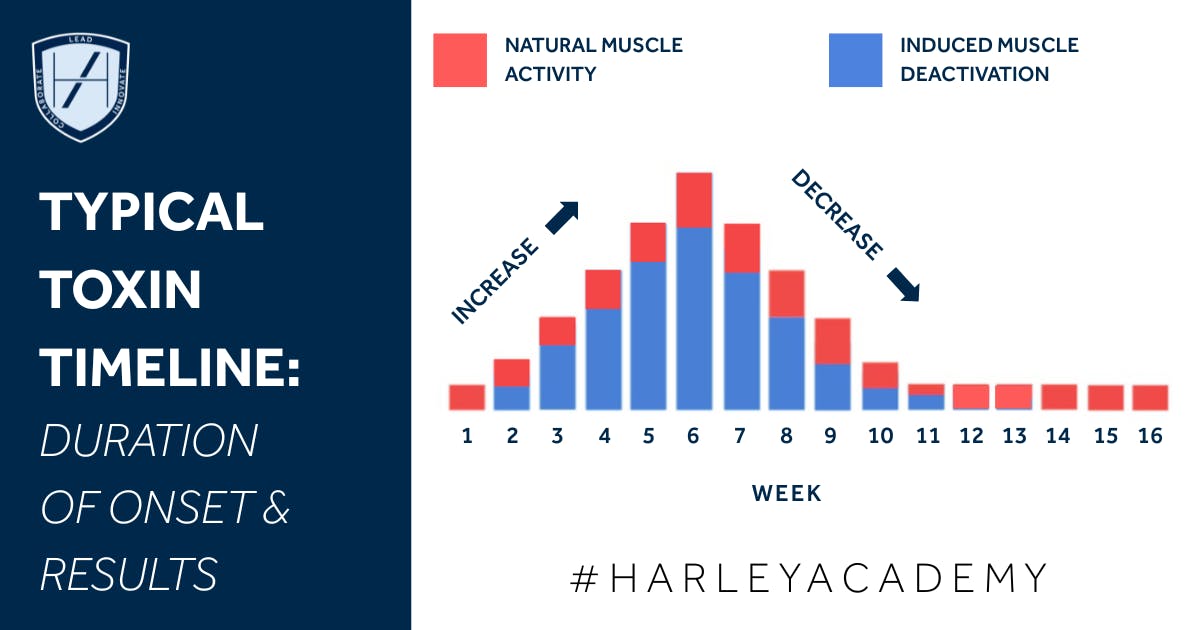
The ‘toxin timeline’ - how long it takes to take effect then wear off - is unique to each individual as well as the product being used.
Using Botox as an example, from a clinical perspective, when you inject into a treated upper face muscle:
- Uptake into the nerve terminal occurs over minutes to hours, and full synaptic blockade may take days
- Onset of any visible effect is typically within 3-7 days, with the full visible result becoming noticeable at around 10-14 days
- General treatment duration in upper face indications is typically around 3-4 months, or 12-16 weeks. However, this can be longer or shorter, and will vary by individual.
The practical takeaway here for aesthetic practitioners is that you would usually expect to re-treat around 12-16 weeks in a typical patient. But you must anticipate patient variability based on muscle mass, activity level, metabolism, injection technique, and other factors.
Below is a handy diagram showing the typical timeline and duration of a standard upper face Botox treatment.
How do GLP-1 receptor agonists work?
GLP-1 receptor agonists (RAs), such as Ozempic and Mounjaro, are increasingly used to manage type 2 diabetes and obesity.
They produce effects including:
- Decreased appetite
- Slower gastric emptying
- Increased insulin secretion
- Weight loss, including lean-mass reduction.
From an aesthetic perspective, this matters because:
- GLP-1 RAs influence body composition, reducing lean mass, which might alter diffusion or uptake of injectables
- They impact systemic metabolism which may alter the pharmacokinetics or pharmacodynamics of other drugs
- As more patients on GLP-1 therapy request cosmetic injectables, including botulinum toxin, the potential for interaction becomes clinically relevant.
Study finds Botox wears off quicker in GLP-1 patients
In October 2025, a paper entitled “Computational modelling the impact of GLP-1 receptor agonists on botulinum toxin A: Evidence for reduced treatment durability across neurologic and aesthetic indications”, was published online.
This digital version was made available ahead of its print publication, scheduled for January 2026.
The study authors, including Eqram Rahman and Jean DA Carruthers, built a computer simulation model using the AesthetiSIM platform, to generate 25,000 virtual patients.
This model encompassed two primary treatment domains: chronic migraine (n ≈ 20 000) and masseter prominence (n ≈ 5 000) to reflect both medical and aesthetic uses. The masseter group was considered ‘cosmetic’ as the treatment was for jawline slimming.
Their virtual patients were randomly assigned to control or various GLP-1 RA exposures:
- Semaglutide
- Tirzepatide
- Liraglutide
- Dulaglutide.
Three mechanistic domains that might influence BoNT-A durability were then modelled:
- Synaptic modulation via GLP-1-related cAMP/PKA mediated SNAP-25 phosphorylation
- Lean-mass decline altering diffusion/kinetic parameters
- Metabolic variability reflecting diabetic or rapid weight-loss phenotypes.
Simulated patient findings showed reduced toxin duration
Using this simulation, they reported the following findings:
- Migraine domain patients saw their mean BoNT-A duration decline from ~14.0 ± 2.3 weeks in controls to 12.6, 12.5, 12.2 and 11.8 weeks across GLP-1 exposures (all p < 0.001; hazard ratios 1.54-1.95)
- Masseter domain patients saw their mean duration fall from ~20.1 ± 2.9 weeks in controls to 17.3, 17.0, 16.7 and 16.2 weeks (hazard ratios 1.72-2.08)
- Sensitivity analysis suggested ~55% of the reduction in duration was attributable to synaptic modulation, ~30% to lean-mass decline, and ~15% to metabolic variability.
Essentially, the toxin was less effective and the effects wore off quicker in Botox patients on GLP-1 weight loss medications. This tallied with anecdotal reports some clinicians have received.
As this data is based on a simulation, not a patient-cohort or randomised clinical trial, the authors stressed its limitations. They called for experimental validation via neuronal culture assays or prospective patient cohorts, before modifying treatment intervals or dosing practice.
What this study means for aesthetic practitioners
As an aesthetic practitioner injecting botulinum toxin in patients who may be taking Ozempic, Mounjaro or other GLP-1 RAs, we know what you’re thinking. ‘What does this mean for me? Should I adapt my treatment, and if so, how?’
We advise you to keep the following points in mind…
1. The evidence on Botox and GLP-1 interactions is preliminary and simulation-based
This is not clinical empirical data in actual patients that’s showing shorter duration of botulinum toxin in GLP-1-treated individuals. It’s a sophisticated computational simulation. As such, any change in practice must be cautious. The authors themselves note that experimental validation is required.
We must acknowledge the limitations of this study. This includes variations between the type of GLP-1 agent, dose, duration of therapy or underlying patient phenotype such as body mass and metabolic rate, patient’s age, muscle activity, previous toxin treatments, and/or dosage.
At this stage, this emerging data should be seen as being hypothesis-generating, rather than practice-changing.
2. The magnitude of effect predicted is modest but clinically relevant
In the jawline slimming aesthetic cohort, this model suggests a drop in mean duration from about 20 weeks to approximately 16-17 weeks for GLP-1 users. That’s a reduction of approximately 3-4 weeks in average durability.
If confirmed, this is relevant to scheduling toxin review appointments and managing patient expectations. It’s certainly something to bear in mind when consulting your patients, particularly where they may have been receiving botox prior to starting weight loss medication. We’ll explore this further in a moment.
In the migraine domain - medical treatment, so less directly relevant to aesthetic practitioners - the reduction was from ~14 weeks to ~11.8 weeks. Therefore, the pattern suggests there is a possible interaction across indications.
3. Possible mechanisms make clinical sense
The simulated mechanisms outlined in this new research are plausible:
- GLP-1 receptor agonism may up-regulate synaptic recovery or alter SNAP-25 phosphorylation, thereby shortening the effective blockade period of BoNT-A
- Lean-mass decline is common in GLP-1 induced weight loss and could alter diffusion or the volume of distribution of injected toxin, reducing dwell-time in muscle tissue
- Metabolic alterations, such as changes in clearance and/or muscle repair dynamics, may accelerate neuromuscular recovery.
From a physiological standpoint, these mechanisms align with what we know about BoNT-A kinetics and muscle physiology. For example, uptake is activity dependent and clearance is via nerve terminal and proteasomal pathways.
All this backs the hypothesis that a GLP-1 RA may shorten the duration of effect for botulinum toxin is biologically plausible.
4. Appointments and informed consent for toxin patients on GLP-1s
In light of this modelling, consider the following practical points for your toxin patients using drugs such as Ozempic, Mounjaro, etc.
- Ensure they’re using an authentic, approved version of the drug from a reputable provider, given fake and unapproved versions are widespread
- Anticipate a somewhat shorter longevity of botulinum toxin effect
- Adjust your clinical consultation accordingly. Be explicit in informed consent that individual response may vary, and that concurrent pharmacotherapy, such as GLP-1 RA, might influence duration of their results
- You may wish to re-treat at their review appointment
- Schedule their next appointment for 12-14 weeks post treatment, rather than 16 weeks
- For regular patients, track how long botox results last for them specifically. Ask them to let you know when they feel that the effects are wearing off, and consider tailoring the interval based on observed pattern rather than strict fixed scheduling, moving forward. You can ask your patients to send you a quick video of them raising their eyebrows then frowning, for example, at various intervals post-treatment, so you can better assess and track this for them.
5. Botox injection technique, dosage and muscle choice remain central
While the GLP-1 interaction is a new variable, the fundamentals of botulinum toxin injections remain unchanged.
The strongest modifiable determinants of effect duration are:
- Accurate placement, at or near the motor end-plate - solid anatomical knowledge is paramount
- Appropriate dosage
- Correct patient selection.
Regardless of whether your patients are using GLP-1 therapy, your standard of care remains. This means optimising your technique first, using evidence-based approaches, such as those taught on Harley Academy’s cosmetic injectables courses. Next, consider the potential impact of systemic pharmacology.
If you notice a pattern whereby any of your patients are consistently seeing results that don’t last as long as they potentially should, you may wish to consider a different product. There are various cosmetic botulinum toxin products available now, including some which claim to have shorter onset times, and deliver longer-lasting results.

Clinical case scenario to test your approach!
Ruth is a 54-year-old female patient who is in general good health but is overweight. Three months ago she started taking semaglutide (Ozempic) for weight management.
She attends your aesthetic clinic for upper face botulinum toxin treatment (Botox), with the aim of reducing her forehead lines and crow’s feet. This is her first cosmetic treatment but, if she likes the outcome, she would like to become a regular patient.
Based on the photo of Ruth above, and the information provided:
- What would you ask her during consultation, outside of the standard questions?
- Is there any additional information you would note in her patient file as part of gaining her informed consent?
- How would you adjust your Botox treatments for her glabella and lateral canthal lines, compared to patients not using GLP-1s?
- When would you book her review appointment for?
- Is there anything else you would explain to her, relating to her aftercare, outside of the standard protocols?
Scroll down to see how we’d deal with Ruth’s case!
1. What would you ask her during consultation, outside of the standard questions?
You should ask if she’s using the GLP-1 through her primary care doctor, or through a private provider. This is to check that she’s not accessing a ‘black market’ version of this prescription medication, where interactions may be more unpredictable.
You may also wish to ask her if she’s aware of any potential interactions between Ozempic and Botox.
2. Is there any additional information you would note in her patient file as part of gaining her informed consent?
Detail what you have explained to her, regarding the potential for shorter lasting Botox results due to her Ozempic use, and that she has confirmed her understanding. This should include that any difference is thought to be around 3-4 weeks less, and that how long toxin lasts always varies from person to person. This conversation, and the detailed recording of it, are important for managing Ruth’s expectations.
3. How would you adjust your botox treatments for her glabella and lateral canthal lines, compared to patients not using GLP-1s?
Assuming you're using appropriate dosing, optimal, evidence-based Botox injection techniques, and are confident in delivering the neurotoxin to its intended anatomical targets, you should not adjust your treatment approach based solely on her use of weight loss medication.
4. When would you book her review appointment for?
The review appointment should be booked for the standard 2-4 weeks post treatment. What may vary is her next Botox appointment. You may wish to schedule this for 12-14 weeks after their initial treatment.
5. Is there anything else you would explain to her, relating to her aftercare, outside of the standard protocols?
Consider asking her to monitor her response to treatment, letting you know when she believes she’s no longer seeing the full effect. Ask if she can send you a video of her raising her eyebrows then frowning, 2-4 weeks after her review appointment if you opted to re-treat. Then, again when she sees the treatment wearing off.
This will allow you to better gauge her unique toxin timeline. Once you’ve treated her a few times, you’ll be able to determine any patterns, and schedule her appointments accordingly. You may also wish to consider dosage or product adjustments at this stage, just as you would for anyone displaying shorter lasting-than-usual results.
Lastly, remember that weight, use of weight loss drugs and signs of ageing can all be sensitive topics. Always approach these discussions from a medical and empathetic perspective. Try to use softer, accessible language so your patients feel cared for, rather than judged.
All information correct at time of publication
Download our full prospectus
Browse all our injectables, dermal fillers and cosmetic dermatology courses in one document
By submitting this form, you agree to receive marketing about our products, events, promotions and exclusive content. Consent is not a condition of purchase, and no purchase is necessary. Message frequency varies. View our Privacy Policy and Terms & Conditions
Attend our FREE open evening
If you're not sure which course is right for you, let us help
Join us online or in-person at our free open evening to learn more
Our Partners













STAY INFORMED
Sign up to receive industry news, careers advice, special offers and information on Harley Academy courses and services